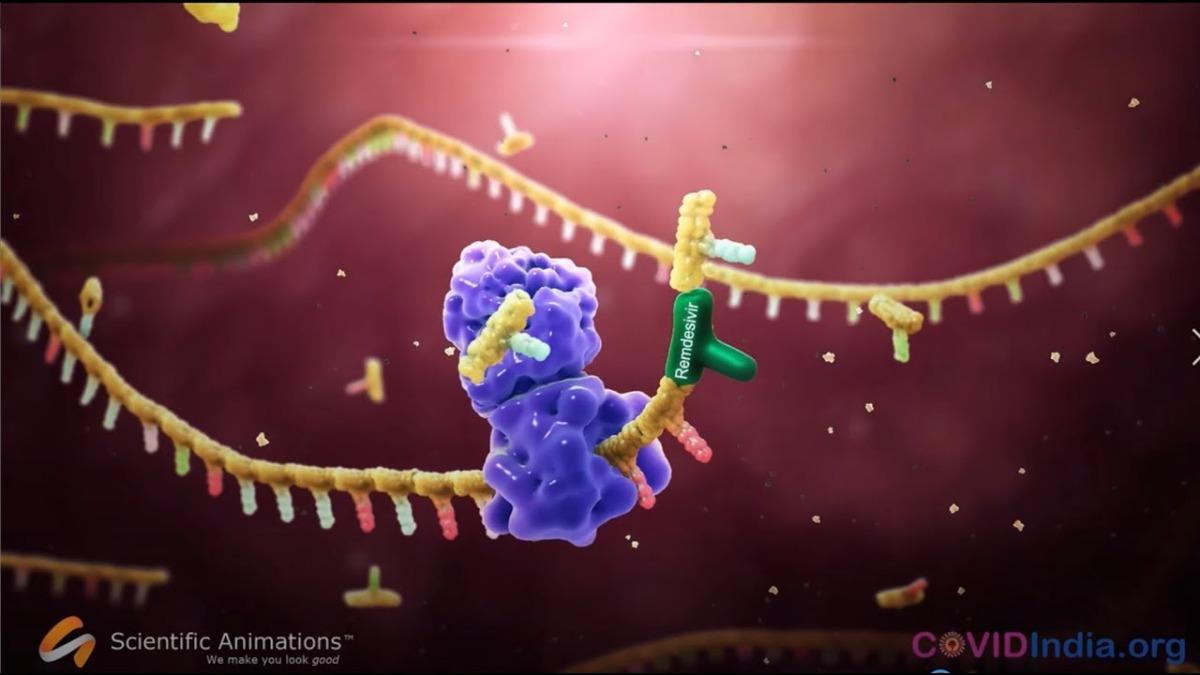
The Food and Drug Administration (FDA) has approved the antiviral Veklury (remdesivir) for treatment of COVID-19 requiring hospitalization in adults and pediatric patients 12 years of age and older who weigh at least 40 kilograms (kg).
How does remdesivir work?
Veklury is a SARS-CoV-2 nucleotide analog RNA polymerase inhibitor. It stops the virus from replicating. In order to make copies of itself, the coronavirus has to copy its genome, which is made up of building blocks called nucleosides that are linked together like Legos. Remdesivir blocks this process. It “looks” like a nucleoside (or 'Lego" block), so the virus inserts it into its genome. This stops the genome copying process in its tracks, and prevents the virus from replicating.
How is remdesivir administered?
It is administered by intravenous infusion as a single loading dose on day 1 followed by once-daily maintenance dosing beginning on day 2 for five to 10 days, depending on clinical severity and response.
What studies have been conducted?
The approval of Veklury was supported by the FDA's analysis of data from three randomized, controlled trials in patients hospitalized with mild to severe COVID-19.
Can younger children get it?
Yes, most children under 12 can still receive remdesivir if they are hospitalized and have severe COVID19. Up to know it has been used in the Pediatric intensive care units. To ensure continued access to the pediatric population previously covered under the emergency use authorization (EUA), the FDA revised the EUA for Veklury to authorize the treatment of suspected or laboratory confirmed COVID-19 in hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized patients younger than 12 years weighing at least 3.5 kg.
Watch out for fake treatments.
The FDA has not approved any over-the-counter medications or products that claim to protect people from COVID19 and/or influenza.
If your child has symptoms of COVID19, call your pediatrician.
Source: https://www.fda.gov/science-research/pediatrics/aap-news-fda-updates
